Background: Venetoclax has shown a promising benefit in patients (pts) with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). There is paucity of information about the efficacy and risk of GVHD with alloSCT post venetoclax -based therapy.
Methods: We conducted a retrospective analysis of 35 AML/ALL pts who received alloSCT following venetoclax-based therapies between 2013-2018 at MD Anderson Cancer Center.
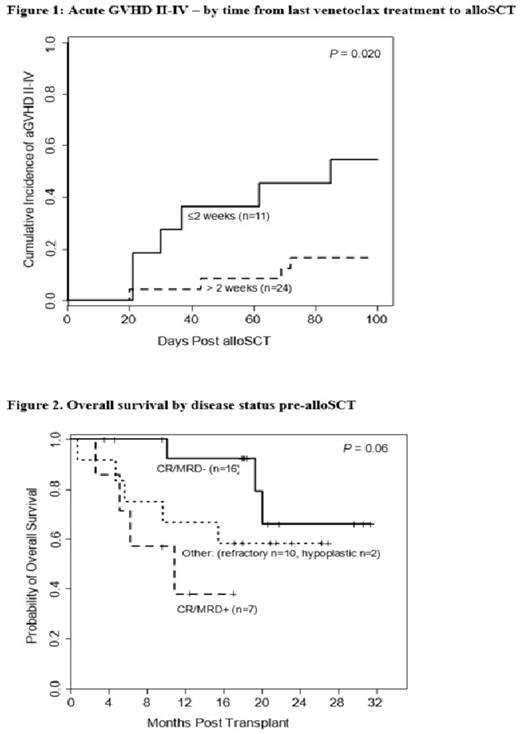
Results: Median age at alloSCT was 60 years and 23 (66%) pts had an age-adjusted HCT-CI score > 3. Disease diagnosis - AML (n=31; 89%), ALL (n=4; 11%). Disease status in pts with AML at study entry was CR1 (n=11; 35%), CR2/CR3 (n=6; 19%), Cri (n=2, 6%), hypoplastic marrow (n=2; 6%) or refractory disease (n=10; 32%). ALL pts were in CR1 (n=2) or CR2 (n=2). Sixteen (46%) pts were MRD-negative by flow cytometry on marrow samples pre-alloSCT, 7 (20%) were MRD-positive, and 10 (29%) had active disease; 2 pts had hypoplastic marrow. Median lines of prior therapies was 2 (range 1-7) and 6 (17%) pts had failed a prior alloSCT. Disease risk index was intermediate (n=18 of 34; 53%) or high/very-high (n=16 of 34; 47%). Venetoclax was provided in combination of hypomehylating (HMA) agents or other chemotherapies in 26 (74%) and 9 (26%) pts, respectively. Median duration of venetoclax-based treatment was 2.0 months (range 0.5- 4.6). Most pts (83%) continued their therapy as a bridge to alloSCT. Venetoclax was discontinued in 6 (17%) pts due to adverse events (n=4) or progression (n=2). Conditioning regimens were melphalan-based reduced intensity (n=26, 74%), or busulfan-fludarabine regimens (n=9; 26%). Donor source was matched unrelated (n=13, 37%), or related (n=9; 26%); haplo (n=12; 34%) and mismatched unrelated (n=1; 3%). Cell source was from peripheral blood (n=24; 69%) or bone marrow (n=11; 31%). GVHD prophylaxis consisted of tacrolimus with either post-transplant cyclophosphamide in 25 (71%) pts or methotrexate in 10 (29%) pts. Seven (20%) pts (3 with active disease, 2 MRD-positive and 2 were MRD-negative pre-alloSCT)) received maintenance therapy with agents other than venetoclax (Vidaza=2, Sorafenib =2, SGI =1, Ponatinib =1, Asparginase = 1) post alloSCT at a median of 3 months (range, 2-4 months). Median time to ANC >500 was 15.5 days (range, 10-24), and platelets counts >20 k was 22.5 days (range, 11-46). The cumulative incidence (CI) of acute 2-4 and 3-4 GVHD was 29% and 9%, respectively. Risks associated with 100 day-acute 2-4 GVHD were evaluated in univariate analyses. Factors evaluated were age, HCT-CI, # prior lines of therapies, prior alloSCT, type GVHD prophylaxis, donor type, cell source, female donor to male recipients, prior PD-1 therapy (n=3) , types of combination of venetoclax duration of treatment and timing of last venetoclax treatment to alloSCT. Only venetoclax timing to alloSCT was associated with significant risk. The CI of acute 2-4 GVHD in patients who discontinued venetoclax ≤ 2 weeks (n=11, 31%) vs > 2 weeks (n=24, 69%) was 55% vs 17% (P = 0.020; Figure 1), respectively [HR 0.24, 95% confidence interval 0.07-0.80; P = 0.020). . With a median follow up among surviving pts of 18.4 months (range 3.5-31.4), the 1-year rates of OS, PFS, and NRM were 72%, 57% and 12% respectively. Twelve patients died: 8 due to progression, 2 of GVHD, 1 of infection and 1 of unknown cause. Disease status was the only predictor of OS in univariate analysis. Pts who were MRD-negative pre-alloSCT had a 1-year OS of 92% while the 1-year OS in pts who were CR/MRD+ and those with active disease was 38% and 70%, respectively (Figure 2). No factor evaluated was significantly associated with NRM. Conclusions: Our data show that the use of venetoclax-based therapy as a bridge to alloSCT does not increase the risk of NRM or delayed engraftment. It does improve OS by inducing CR/MRD-negativity in high-risk acute leukemia. It may be prudent however to discontinue therapy > 2 weeks before alloSCT in order to avoid a higher risk of acute GVHD.
Jabbour:BMS: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding. Daver:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Konopleva:Cellectis: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; F. Hoffmann La-Roche: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Sanofi: Research Funding; Eli Lilly: Research Funding; Rafael Pharmaceutical: Research Funding; Ascentage: Research Funding; Agios: Research Funding; Amgen: Consultancy; Genentech: Consultancy, Research Funding; Kisoji: Consultancy; Ablynx: Research Funding; Stemline Therapeutics: Consultancy, Research Funding; AstraZeneca: Research Funding; Calithera: Research Funding; Forty-Seven: Consultancy, Research Funding. DiNardo:Novartis: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria; Notable Labs: Membership on an entity's Board of Directors or advisory committees; Syros: Honoraria; Calithera: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; MedImmune: Honoraria; Jazz: Honoraria; Agios: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding. Ravandi:Orsenix: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Xencor: Consultancy, Honoraria, Research Funding. Kadia:Pulmotec: Research Funding; Astra Zeneca: Research Funding; Genentech: Honoraria, Research Funding; Cellenkos: Research Funding; Celgene: Research Funding; Amgen: Research Funding; Ascentage: Research Funding; Novartis: Honoraria; Incyte: Research Funding; JAZZ: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Astellas: Research Funding; Cyclacel: Research Funding; Pfizer: Honoraria, Research Funding. Alousi:Incyte: Honoraria, Research Funding; Alexion: Honoraria; Therakos: Research Funding. Oran:Arog Pharmaceuticals: Research Funding; Celgene: Consultancy; ASTEX: Research Funding. Kebriaei:Amgen: Other: Research Support; Pfizer: Other: Served on advisory board; Ziopharm: Other: Research Support; Kite: Other: Served on advisory board; Novartis: Other: Served on advisory board; Jazz: Consultancy. Popat:Bayer: Research Funding; Novartis: Research Funding. Kantarjian:Aptitute Health: Honoraria; BioAscend: Honoraria; Adaptive biotechnologies: Honoraria; Novartis: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Oxford Biomedical: Honoraria; Immunogen: Research Funding; BMS: Research Funding; Ascentage: Research Funding; Amgen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Jazz: Research Funding; Janssen: Honoraria; Delta Fly: Honoraria; Daiichi-Sankyo: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Champlin:Omeros: Consultancy; Actinium: Consultancy; DKMS America: Membership on an entity's Board of Directors or advisory committees; Cytonus: Consultancy; Genzyme: Speakers Bureau; Takeda: Patents & Royalties; Johnson and Johnson: Consultancy. Khouri:Bristol Myers Squibb: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal